Validation of Combs prognostic scoring system in Indian recurrent glioma patients treated with re-radiation
Article information
Abstract
Purpose
Retrospective audit of recurrent glioma patients treated by different fractionation schedules and to validate the modified Combs prognostic score in Indian patient cohort.
Materials and Methods
Between Jan 2009 and June 2022, 66 recurrent gliomas patients treated with standard adjuvant treatment—radiation (RT) ± temozolomide (chemotherapy)—and re-treated with RT (± chemotherapy) were categorized as per modified Combs prognostic criteria and outcomes were compared.
Results
Sixty-six patients with recurrent gliomas who received reirradiation (re-RT) were audited—53% males; 61% Karnofsky performance status (KPS) ≥80 at time of re-RT; median age 41.5 years (range, 6 to 70 years); 67% <50 years; primary histology low-grade glioma in 33% ; grade III 27%, grade IV 40%; initial median dose of 60 Gy equivalent dose in 2 Gy fractions EQD2; maximum safe resection at recurrence 41%; mean and median follow-up 78 ± 51 months and 66 months. Mean time interval between RT was 46.4 ± 39 months. Mean planning target volume (PTV) volume in conventional RT (Conv-RT), hypofractionated RT (Hypo-RT), and ultra-hypofractionated RT (UF-RT) was 226.1 ± 140.7 mL, 162.8 ± 123.3 mL, and 143.3 ± 145.8 mL. Mean dose for Conv-RT, Hypo-RT, and UF-RT was 50 Gy (range, 40 to 60), 31 Gy (range, 20 to 40), and 20 Gy (range, 10 to 30). Mean overall survival (OS) in Conv-RT, Hypo-RT, and UF-RT cohort was 18.8 months (range, 2.4 to 76.8); 6.6 months (range, 2 to 17.4), and 13.9 months (range, 3 to 131.9). Median OS as per Combs criteria were 16.6 months (Group a), 24.6 months (Group b), 4.6 months (Group c), and 3 months (Group d). Significant survival benefit was with good KPS score (KPS >80 vs. <80; 20.46 vs. 5.25 months; p < 0.001), patients receiving salvage chemotherapy (20.46 vs. 6.96 months; p = 0.001), and patients received re-RT biological equivalent dose BED3 >80 Gy (16.62 vs. 5.48 months; p = 0.03). Median OS in our patient cohort and Combs cohort in Group a was 16.6 and 19.5 months; Group b was 24.6 and 11.3 months; Group c was 4.7 and 8.1 months, and Group d was 2 and 5.5 months, respectively. Six months survival in our patient cohort and Combs cohort in Groups a, b, c, d were 100%, 92%, 34%, 17% and 94%, 79%, 70%, 41%, respectively. Twelve months survival in our patient cohort and Combs cohort in Groups a, b, c, d were 88%, 74%, 22%, 0% and 88%, 47%, 22%, 7%, respectively.
Conclusion
Modified Combs prognostic factors predicts OS and is applicable in Indian subcontinent patient population.
Introduction
Radiation therapy (RT) remains one of the key treatment modalities in the management of primary central nervous system (CNS) tumours [1]. It is indeed a well-known fact that the natural history of glioma follows a very high tendency for local recurrence [1]. Despite a multimodality approach with surgery, RT, and chemotherapy, the relapse rates are high [2,3]. There is no standard or recommended optimal salvage treatment protocol in such a scenario. Reirradiation (re-RT) for such recurrences have always been extremely challenging in view of the high risk for toxicities [4,5]. Modern RT techniques and better understanding of the radiobiology of tumors and normal tissues in CNS, has made it possible to overcome few of these challenges. Salvage surgery in most cases is difficult and appears to be feasible only for selected cases. Patient selection for re-surgery has been assessed with help of a prognostic score developed by Park et al. [6].
Re-RT is a viable option however radiation toxicity is a major concern [4,5]. Over the past decade, lot of interest has been noted in the role of re-RT for recurrent gliomas using modern photon RT techniques. Multiple factors are involved in determining outcome of patients with recurrent gliomas. Patient selection for re-RT is the major concern in predicting outcome. Comb et al. [8] developed and validated a prognostic score for recurrent gliomas, which helped predict the survival benefit following re-RT. This prognostic score identifies three main factors influencing survival—primary histology, age, and time interval between primary RT and re-RT. The “Heidelberg Combs prognostic score” was later modified by including other significant factors like re-resection, Karnofsky performance status (KPS), and tumour volume [8,9]. New Combs prognostic score is a useful tool to predict the benefit of re-RT in recurrent glioma patients [8-10]. However, this prognostic scoring system needs validation in different patient population.
This present retrospective audit of Indian recurrent gliomas patients treated with re-RT were analyzed. The aim of this analysis is to validate the Combs prognostic scoring system in Indian (Asian) patient population, and also evaluate the outcome after re-RT with different fractionation schedule.
Materials and Methods
During 2009 to 2022, 66 recurrent gliomas after radical RT (with or without concomitant chemotherapy) re-treated with RT (±concomitant chemotherapy) were audited. Patients were accrued from two centres and retrospective analysis were done. Patients with radiological diagnosis of glioma who were treated with surgery followed by standard radical intent RT with or without chemotherapy (temozolomide [TMZ]), had recurrence and re-treated with RT were accrued in this audit. Patient and treatment related data were collected from institutional electronic medical records. Patients were categorized according to the Modified Combs prognostic factors—primary histology, KPS, age, time between primary RT and re-RT, re-resection status and tumor volume—into four groups (a, b, c, d) and their outcomes were compared.
During regular follow-up, radiological evaluation was done for evaluating response to treatment or recurrence. In cases of recurrence, surgery option was considered as the standard option and in cases where surgery was not possible or after re-surgery where there was residual disease, in those situations re-radiation was considered. Sixty-six patients treated with re-radiation were accured in the present analysis. Re-radiation dose, volume and radiation techniques were considered as per available protocol. Magnetic resonance imaging (MRI) scan with contrast and magnetic resonance perfusion was done and fused with planning computed tomography (CT) scan. Clinical target volume (CTV) was considered as the pre-surgery recurrent disease volume along with T2 flair changes. However, post-RT changes in T2 flair sequences were not included in the CTV. Previous plan when available was fused with the present plan. Planning target volume (PTV) margin of 3–5 mm was considered. Radiation dose was considered as per the volume of the target (PTV), previous radiation dose and gap between RT and re-RT. Radiation technique used were three-dimensional conformal radiation therapy (3DCRT), volumetric-modulated arc therapy (VMAT), tomotherapy and robotic radiosurgery depending upon availability of the facility and volume of the target (PTV). Patient related factors, treatment related factors, and pathology/molecular parameters were documented for analysis. Starting date of re-radiation therapy were considered for calculation of overall survival (OS) and progression-free survival (PFS) after re-radiation therapy. Factors influencing survival functions were analysed.
Patients were grouped as per Combs criteria depending upon age, performance status, histology, re-surgery, gap between RT and re-RT. Analysis of outcome as per Combs grouping were done and compared with Comb’s published data. Molecular parameters (IDH1, 1p19q, ATRX) when available were documented. An attempt was done to evaluate the applicability of Comb’s criterias in Indian patient population and select suitable additional criterias for Indian patient population.
1. Statistical analysis
Patient and treatment related parameters were collected using IBM SPSS 26.0 (IBM, Armonk, NY, USA). For continuous variables, results are given in mean ± standard deviation, and categorical variables in percentages. Categorical variables were compared with chi-square test. OS was calculated from date of re-RT to date of last follow up or death. PFS was calculated from the date of re-RT to date of progression (progression was defined as radiological or clinical progression). Radiological response and progression were confirmed by institutional neuro-radiologist. Survival rates were calculated using Kaplan-Meier methods and compared using log-rank test. p-value <0.05 was considered statistically significant.
Results
1. Demography
In our cohort, 66 patients with recurrent gliomas who received re-RT were included, among whom 53% were males and 40 patients (61%) had KPS ≥80 at time of re-RT (median age of 41.5 years; range, 6 to 70 years) (Table 1). Sixty-seven percentage of patients were less than 50 years. Primary histology was low-grade glioma in 22 (33%), Grade III in 18 (27%) and Grade IV in 26 (40%) patients. Patients had received an initial median dose of 60 Gy equivalent dose in 2 Gy fractions (EQD2). Mean time interval from initial RT to re-RT was 46.4 ± 39 months and median time interval was 34 months. Forty-nine patients (74%) had progression-free interval more than 12 months. At recurrence, 37 patients had radiological high-grade glioma, 23 patients had biopsy proven Grade IV glioma, one patient had biopsy proven Grade II, and five patients had biopsy proven Grade III glioma. Maximal safe resection prior to re-RT was performed in 27 (41%) patients and 37 (56%) patients were treated with radiological diagnosis. Mean follow-up was 78 ± 51 months and median follow-up was 66 months.
2. Re-RT treatment characteristics
At the time of re-RT, 31 (47%) patients received conventional fractionation RT (Conv-RT; 1.8–2 Gy per fraction), 12 (18%) received moderately hypofractionated RT (Hypo-RT; 2–4 Gy per fraction), and 23 (35%) patients received ultra-hypofractionated RT (UF-RT; >4 Gy per fraction) (Table 2). Median PTV volume at re-RT was 163.40 mL (range, 4.5 to 582 mL). Median and mean PTV volume in patients receiving Conv-RT was 180 mL (range, 24 to 574 mL) and 226.1 ± 140.7 mL; median and mean PTV volume for patients receiving Hypo-RT was 156.9 mL (range, 27.5 to 387.9 mL) and 162.8 ± 123.3 mL. UF-RT cohort had median and mean PTV volume of 84.6 mL (range, 4.5 to 582 mL) and 143.3 ± 145.8 mL, respectively (Table 3).
Mean dose for Conv-RT, Hypo-RT, and UF-RT cohort was 50 Gy (range, 40 to 60 Gy), 31 Gy (range, 20 to 40 Gy), and 20 Gy (range, 10 to 30, Gy), respectively. Mean OS in Conv RT, Hypo-RT, and UF-RT cohort was 18.8 months (range, 2.4 to 76.8 months), 6.6 months (range, 2 to 17.4 months), and 13.9 months (range, 3 to 131.9 months), respectively.
Patients received mean EQD2 of 45.57 Gy, and median BED3 at re-RT was 75.6 Gy (range, 36.7 to 103.7 Gy). Fifty-five percentage patients received BED3 of <80 Gy at re-RT, while 18% received BED >90 Gy. Median cumulative BED3 was 169 Gy (range, 118 to 203.70 Gy). Twenty patients (30%) received 3DCRT, 23 (35%) received intensity modulated radiotherapy (IMRT)/volume modulated radiotherapy (VMRT), and 23 (35%) received stereotactic radiosurgery (SRS)/fractionated stereotactic radiosurgery (fSRS). Twenty-eight patients (42%) received concurrent TMZ, while 38 (58%) received post-RT maintenance/salvage chemotherapy.
3. Re-RT treatment and outcome parameters
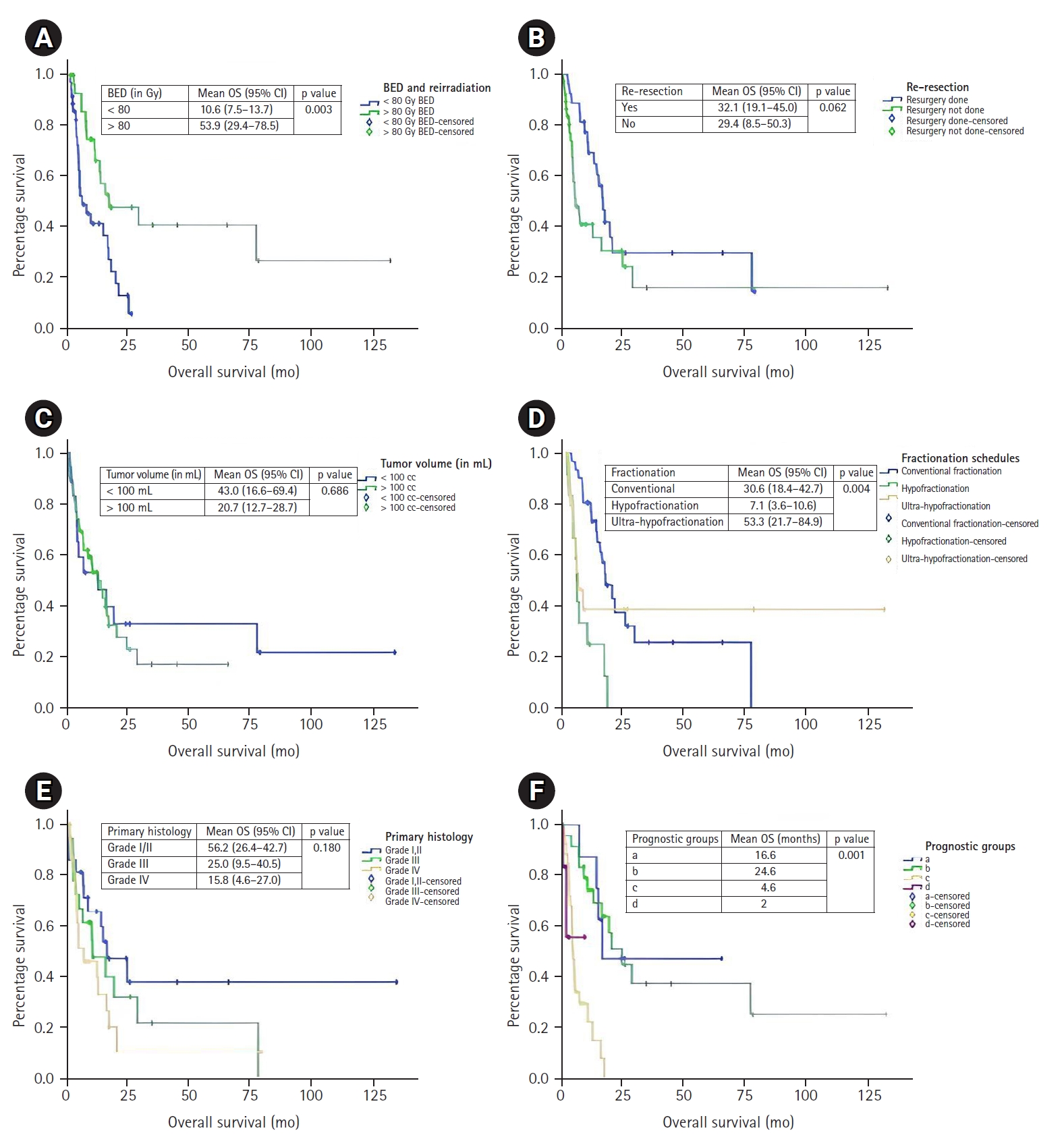
The median OS of the entire cohort after re-RT was 13.0 months (95% confidence interval, 7.6–18.4) after a median follow-up period of 34 months from initial RT (Fig. 1). The median OS of patients when categorized as per Combs criteria were 16.6 months (Group a), 24.6 months (Group b), 4.6 months (Group c), and 3 months (Group d). Univariate analysis was done to identify the prognostic markers as per Combs criteria and additional factors that could have an impact on survival function. Concurrent TMZ, salvage/maintenance chemotherapy, fractionation schedule, BED received at re-RT, PTV, and age were analyzed. Statistically significant higher OS was noted in patients with KPS score >80 (20.5 vs. 5.3 months; p < 0.001). A trend toward improvement in survival was noted in patients who were able to undergo resection (16.6 vs. 5.5 months; p = 0.062). Among other factors, statistically significant OS was noted in patients receiving salvage/maintenance chemotherapy (20.5 vs. 7.0 months; p = 0.001) and those who received re-RT BED3 >80 Gy (16.6 vs. 5.5 months; p = 0.003). We also tested fractionation schedule and survival function was significantly better with Conv-RT schedule. Median OS with Conv-RT schedule was 16.6 months, 4.4 months with Hypo-RT, and 5.2 months with UF-RT (p = 0.004). Nearly half of our patient cohort were aged <40 years, univariate analysis was done with age at treatment cutoff of 40 years. Median OS in patients with age <40 years was 14.2 versus 12.6 months with patients age >40 years, however this was not statistically significant.
4. Univariate analysis as per Combs criteria
Median OS in Grade I/II, Grade III, and Grade IV were 16.6, 10.7, and 7.3 months, respectively (p = 0.180) (Table 4). Median OS in <50 and >50 years were 16.7 and 10.7 months, respectively (p = 0.17) (Figs. 2, 3). Median OS in time interval between RT <12 and >12 months were 7.32 and 15 months, respectively (p = 0.100). KPS >80 and <80 were 20.5 and 5.3 months, respectively (p = 0.001). Median OS in re-resection done and not done patients were 16.6 and 5.5 months (p = 0.062). Median OS in PTV <47 and >47 mL were 13 and 12.9 months, respectively (p = 0.530).
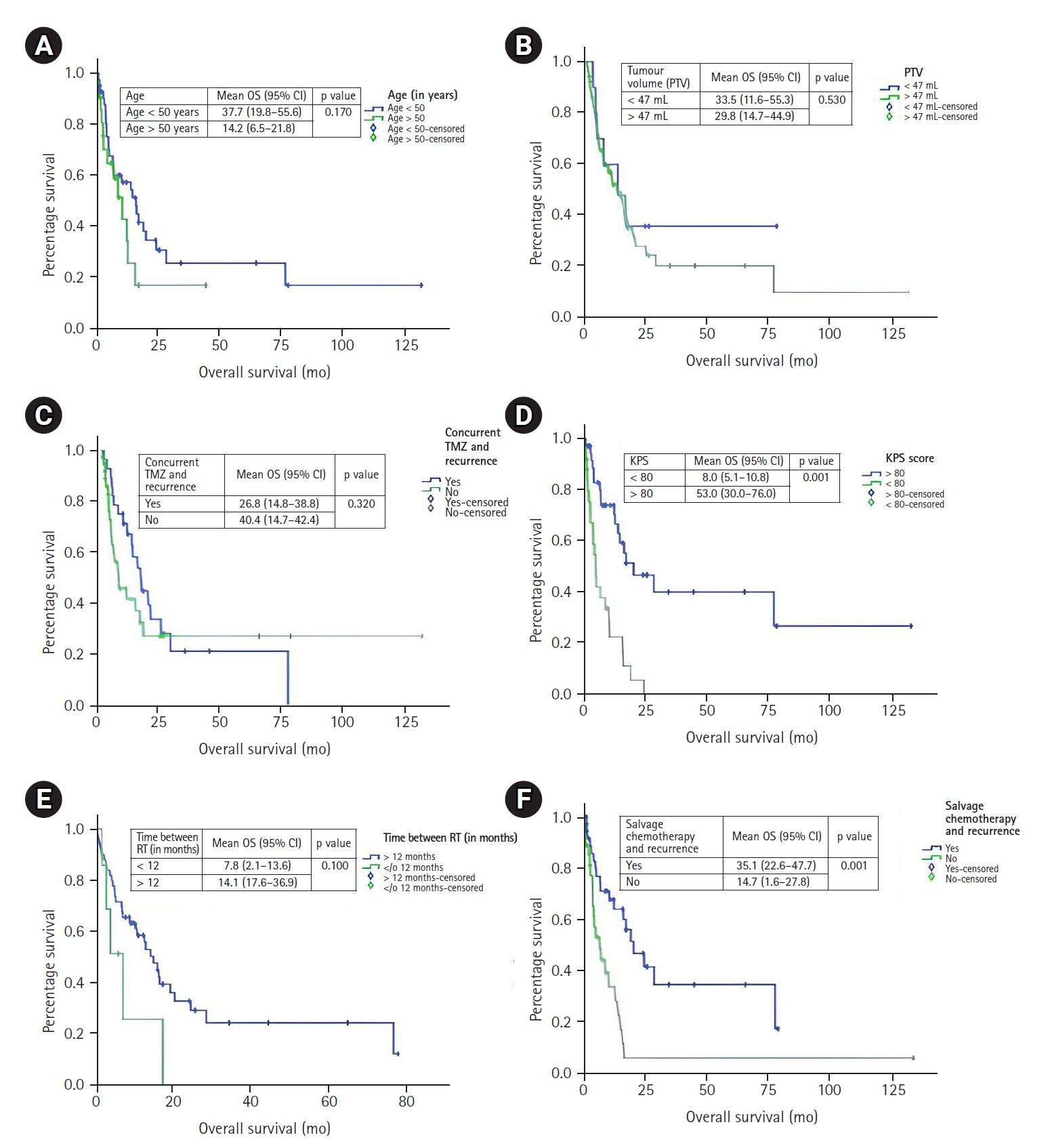
Factors influencing outcome after re-irradiation in recurrent gliomas according to Combs criteria: (A) age, (B) tumor volume, (C) concurrent temazolamide, (D) Karnofsky performance status (KPS), (E) time between RT, and (F) salvage chemotherapy. OS, overall survival; PTV, planning target volume; TMZ, temozolomide; RT, radiotherapy.
5. Univariate analysis additional criteria
Median OS in patients who received concomitant TMZ and no TMZ were 16.4 and 7.3 months (p = 0.320) (Table 5, Figs. 2, 3). Median OS in Conv-RT, Hypo-RT, UF-RT were 16.6, 4.5, and 5.2 months (p = 0.004). Median OS in BED3 at Re-RT <80 and >80 Gy were 5.4 and 16.6 months (p = 0.003). Median OS in PTV >100 and <100 mL were 14.3 and 13 months (p = 0.686). Median OS in age <40 and >40 years were 14.3 and 12.7 months (p = 0.773).
6. Survival function as per Combs scoring system
Median OS in our patient cohort and Combs cohort in Group a was 16.6 and 19.5 months; Group b was 24.6 and 11.3 months; Group c was 4.7 and 8.1 months; and Group d was 2 and 5.5 months, respectively (Table 6). Six months survival in our patient cohort and Combs cohort in Groups a, b, c, d were 100%, 92%, 34%, 17% and 94%, 79%, 70%, 41%, respectively. Twelve months survival in our patient cohort and Combs cohort in Groups a, b, c, d were 88%, 74%, 22%, 0% and 88%, 47%, 22%, 7%, respectively.
Discussion and Conclusion
Brain tumour consists only 3% of all tumors and glioma is most common primary tumour. Though brain tumor occurs in a small proportion of patients, advancements in molecular genetics, surgical and RT techniques, and also radical evolution in radiology and proteomics have established brain tumour treatment as one of the most sophisticated treatment approaches [11]. Standard treatment option in glioma patients is surgery alone or followed by RT or concurrent chemoradiotherapy (TMZ) or observation [2]. In high-grade glioma, “Stupp protocol,” which was established almost 15 years back is still considered the standard of care [2,11]. In high-grade glioma, 80% of the patients have recurrence at the surgical bed, more precisely around 2 cm of the cavity within 1 year after treatment [4,12]. Low-grade gliomas are also having relatively dismal prognosis. One- and 2-year survival in diffuse low-grade gliomas (Grade II) is more than 80%, but if we look at the long-term outcome, 5- and 10-year survival is only 40% and 20%, respectively [13]. Most of these patients recur at the primary site without metastasis. Hence, local treatment is of paramount importance even at the time of recurrence.
Problems tackling recurrent high- and low-grade gliomas are different. High-grade gliomas recur in a very short interval after surgery and RT. Hence, repeat surgery or RT is a challenge. High-grade gliomas recurrence usually occurs at the tumour bed, but in most of the time there is infiltration of the adjacent parenchyma with post-radiation changes. These factors make surgery challenging in recurrent gliomas after RT. In recurrent gliomas, RT ideally should be considered as a valid option.
Low-grade gliomas usually recur few years after the initial treatment. “High-risk” low-grade gliomas have a higher tendency of recurrence and recur earlier than “low-risk” gliomas. Majority of these low-grade glioma’s recurrence are clinically indolent and present with symptoms only when there is extensive infiltration to the adjacent normal tissues. Another significant factor is that at recurrence a good proportion of these initially low-grade glioma patients have transformed to high-grade glioma. In both the situation, surgery is the definitive treatment, but complete excision is rarely achieved. Hence focal RT should play an important role for local control and survival advantage.
According to Nider et al. study [14], normal brain has a “maximum limit” of delivering 100 Gy maximum dose in lifetime. All high-grade gliomas had already received more than 60 Gy and low-grade gliomas 50–54 Gy. Most of the recurrence is high-grade glioma (de novo or transformed) have received 60 Gy BED2 dose and eligible to receive only 40–45 Gy. This dose may not be adequate for recurrent high-grade gliomas and hence the outcome even after re-RT is dismal. Re-RT invites post-radiation necrosis, edema and related late toxicities. Time gap between RT and re-RT may be critical, as “deliverable” total RT dose may be higher with longer time gap between RT and potential risk of necrosis will also be lower. During planning and dose delivery previous plan dose parameters are preferably included, “overlap” and “non-overlap” region defined. Dose planned as per previous received dose to the region and gap between previous RT. Histopathology and molecular parameters after recurrence may also be important factor regarding RT effectiveness. Dose and fractionation schedule of radiation at re-RT may impact the response to treatment [2]. Multiple factors influence the selection of recurrent glioma patients for re-RT, and hence various different scoring systems were introduced for appropriate case selection and predict outcome after re-RT [8-10]. There is no randomized study comparing re-RT therapy in recurrent glioma with other treatment modalities. Most of the publications regarding re-RT in recurrent glioma are single institutional retrospective series [15-18]. Among them, 76% publications are from the United States universities (only four centers) and rest 24% from European centers. Only 4% of these series are from Asia and there is little published literature from Indian patient cohort. Role of re-RT therapy in recurrent gliomas are still evolving with highest level of evidence is only2A. There is a need to have clinical outcome data in recurrent gliomas patients from different geographical population [11,19-21].
Carson et al. [7] had proposed a recursive partitioning analysis (RPA) class for recurrent gliomas. They proposed seven RPA classes for recurrent gliomas based on initial histology, age, KPS, and corticosteroid for prognostication of recurrent glioma patients. This RPA classification allowed comparisons of outcome between different phase II clinical trials and to define enrollment criteria for accural in clinical trials. Mean OS in RPA-1 was 25.7 months, RPA-2, 17.2 months; RPA-3, 3.8 months; RPA-4, 10.4 months; RPA-5, 5.6 months; RPA-6, 6.4 months, and RPA-7, 4.9 months. This was the first attempt to define prognostic groups in recurrent gliomas based on patient related factors. Combs et al. [8] made a structured attempt to define prognostic groups in recurrent gliomas after treatment with re-RT. First attempt with 82 recurrent gliomas did not show any significant prognostic group [10]. Then analysis with larger patient cohort (n = 232) suggested histology, age, and time between RT and re-RT as independent prognostic factor in univariate analysis. Scoring done based on histology (GBM-2, AA-1, Grade 2-0), age (score 0 of <50 years, score 1 of >50 years), gap between RT (1 of <12 months, 0 of >12 months). Higher score had poorer prognosis and lower scores predicted better outcome. Twelve months survival in score 0 was 50% and in score 4 was only 8%. Radiation dose, PTV volume, molecular markers did not show prognostic significance. Based on this scoring system, score of 0–2 suggested for aggressive treatment and score more than 4 considered for palliative treatment [10]. Kessel et al. [9] analyzed the same patient cohort with longer follow-up and more patient numbers. In this cohort, apart from primary histology, age, time gap between RT few other factors such as KPS, surgery for recurrence and tumour volume were also shown to be statistically significant. MGMT (O6-methylguanine-DNA methyltransferase) methylation status was also significant in univariate analysis. These new factors were included and a modified Combs criteria were proposed for prognostication of recurrent gliomas treated with re-RT. Modified Combs criteria groups were a, b, c, d and the median OS was 19.5, 11.3, 8.1, 5.5 months, respectively. Six months and 12 months survival were 94%, 79%, 70%, 41% and 88%, 47%, 22%, 7%, respectively. Modified Combs prognostic scoring system was validated in German and Italian patient cohort [17,18]. This prognostic scoring system was not validated in Asian/Indian subcontinent patient population [14]. PTV was shown to have significant survival correlation. Larger PTV had poorer response to re-RT. PTV <47 mL had significantly better survival [11,19]. Fractionation schedule was based on the PTV. In small volume recurrence (<30 mL) patients may be considered for UF-RT (30–35 Gy per 5 fractopms), intermediate volume recurrence (30–50 mL) for Hypo-RT (35–40 Gy per 15 fractions) and large volume recurrence (>50 mL) planned with Conv-RT (45 Gy per 25 fractions) [14]. With PTV stratification, survival outcome was similar with all the frationation schedules. Patients treated with more than 41.4 Gy at re-RT setting had higher survival function [19]. Interestingly, though time gap between RT had shown to be significant in univariate analysis, it was not significant in multivariate analysis [10,11]. GBM patients recur within 12 months whereas anaplastic astrocytomas recur in more than 12 months. Hence, the histology obscures the impact of time gap between RT on survival function [10]. However, longer time gap between RT allows to deliver higher RT dose. Benefit of concomitant chemotherapy (TMZ) along with RT in case of re-RT of recurrent gliomas is not yet established with meta-analysis [22]. In recurrent gliomas treated with gross total excision, role of RT is not defined. However, as the outcome was significantly better after re-RT in recurrent ependymomas after gross total excision, in recurrent gliomas also usually re-RT is offered [22].
Tolerance of RT at re-RT depends upon re-RT volume, initial RT dose and time gap between RT [14]. Radiation necrosis at 6 months was 7%–10% and 15%–20% at 12 months after re-RT. ROCOCO trial is a dosimetric analysis which suggested that proton therapy may have dosimetric advantages and have the potential to reduce toxicity [23]. However, there is no long-term clinical outcome data available with proton therapy in recurrent gliomas [23]. The aim of the present analysis was to validate the modified Combs scoring system in Indian patient population and compare the outcome with published Western patient cohort. KPS was a significant independent prognostic factor for survival. Resection at recurrence showed a trend towards survival benefit. Patients with recurrent low-grade gliomas had better survival. Younger patients (<50 years) and >12 months gap between RT had better survival. In our cohort of 66 patients, majority were in Groups b and c. In Combs cohort also, majority were in Groups b and c. Hence, the patient selection for re-RT in our retrospective cohort was similar to the Combs cohort. Median OS in Groups a, b, c, d in our cohort and Combs cohort were 16.6, 24.6, 4.7, 2 months and 19.5, 11.3, 8.1, 5.5 months, respectively. The outcome pattern was almost similar in both the cohorts. Better outcome in Groups a and b, whereas poorer in Group c and d. In Group a, 6-month survival in our cohort and Combs cohort were 100% and 94%, respectively, while 12-month survival was 88% in both cohorts. In Group b, similar survival function was 92% and 79% and 74% and 47%, respectively, at 6 and 12 months, in the present patient cohort and Combs data, respectively. Twelve months survival in Group c were 22% in both the cohorts. In Group d, 12-month survival was 0% in our cohort and 7% in Combs cohort. Hence, the pattern of survival in our cohort and Combs cohort were similar. The proportion of patients in Groups c and d (poorer prognosis cohort) were higher in Combs cohort. This suggests that we were more conservative in re-RT and accruing mostly patients with better performance status, smaller volumes and longer gap between RT. Combs prognostic scoring system is valid and applicable in predicting the survival outcome in Indian patient cohort.
In our cohort, a few additional factors were analyzed. Mean OS in patients treated with Conv-RT schedule was 18.8 months, Hypo-RT was 6.6 months, and UF-RT was 13.9 months. Majority of the patients were treated with Conv-RT schedule and this cohort had significantly better survival function. Patients with relative larger volume and longer disease-free interval (DFI) were planned with Conv-RT schedule. More number of recurrent low-grade gliomas were treated with Conv-RT schedule. Significant benefit with Conv-RT schedule may be due to selection bias with a larger number of recurrent low-grade gliomas and longer DFI of the patients in this cohort. Retrospective published series suggested that there may not be any difference in outcome with different fractionation schedules. Comb criteria was developed for patients treated to 36 Gy in 2 Gy per fraction, and the modified Combs-Kessel prognosis score was for 14–60 Gy in Conv-RT. Our data shows 35% of re-RT with UF-RT and good mean OS of 13.9 months compared to median OS of 5.2 months with Hypo-RT schedule. It means we have 50% really good cohort of patients in ultrahypofractionated regimen also who are surviving long. Infact the longest surviving patients are in ultrahypofractionated regime (young patient with small volume recurrent pilocytic astrocytoma after RT treated with UF-RT), whereas, median and mean OS is poor in moderate fractionation. Our UHF regime of 10–30 Gy has to be less toxic than reported series treated with 25 Gy in 5 fractions schedule (median OS was 10 months) [18,19]. Patients treated with more than 40 Gy developed late toxicity, whereas 25% developed late toxicity in 30–40 Gy range (our dose range is 10–30 Gy). A dose of 10–15 Gy seems very low and a dose of 25–30 Gy in UHF may be a good option. Tolerance, completion of treatment and survival may be better with ultrahypofractionated regimes for appropriately selected patients. Higher BED had shown significant survival benefit. Patients with more than 80 Gy BED3 had better survival. This collaborates with the published literature suggesting more than 41.4 Gy in re-RT setting have better survival [9,10]. Our target volume (PTV) was larger than the Combs cohort. The contouring may be more liberal in our series and post primary RT. MRI scan T1 contrast region and T2 flair changes having mass effect were included in the CTV volume. There is no definitive guideline regarding the contouring volume in recurrent setting. Usually the contrast enhancing region and the definite MRI scan T2 flair changes suggesting obliteration of the sulci may be appropriate to include in the CTV and entire T2 flair changes are not included in the CTV. Though the mean volume was higher in our series, there was no significant impact on survival function. Indian patient cohort have one-decade early presentation of high-grade glioma [24]. In our cohort also mean age of presentation was earlier than the Combs cohort. However, early age of presentation did not impact the survival function. There was an attempt to evaluate the need to modify Combs criteria as per Indian patient condition. Age was modified to 40 years, PTV range to 100 mL, but there was no significant impact regarding prognostication. Fractionation schedule and RT dose (BED) were found to be significant contributory factors; however they were not included as the subsite patient numbers were small and the actual impact of these factors needs to be evaluated in a larger patient cohort. Retrospective data and smaller patient cohort are the major limitation of the present study. However, this is one of the few studies evaluating the impact of re-RT in Indian/Asian patient population and validation of Combs prognostic scoring system done [25]. Further study regarding re-RT in recurrent gliomas needs multi-centric approach to accrue a larger number of patients. Molecular parameters need to be included in the analysis.
In conclusion, re-RT is a salvage option in recurrent gliomas with acceptable toxicities for patients with good KPS and treated with high-dose RT (BED >80 Gy). Modified Combs prognostic factors predicts OS and is applicable in Indian subcontinent patient population. Outcome of re-RT in Indian patients with recurrent gliomas were similar with published literature. Fractionation schedule and RT dose have potential to be included in the prognostic criteria. There is a need for prospective multi-institutional collaborative studies to accrue a larger patient cohort with long-term follow-up.
Notes
Statement of Ethics
This study is an observational study. The Amrita Institute of Medical Sciences and Research Institutional ethics committee has confirmed that no ethical approval is required.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author Contributions
Conceptualization, Dutta D. Investigation and methodology, Dutta D, Jose M, Nair H, Sruthi K. Supervision, Dutta D. Writing of the original draft, Dutta D, Jose M. Writing of the review and editing, Dutta D, Jose M, Nair H, Sruthi K, Sasidharan A. Validation, Dutta D, Jose M, Nair H. Formal analysis, Dutta D, Jose M, Nair H. Data curation: Jose M, Nair H. Visualization: Dutta D, Sruthi K. All the authors have proof read the final version.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.